What is Acrylonitrile ( AN )? Hazard Classification, Uses, Risks, and Storage Guidelines
Acrylonitrile is widely used in materials, carbon fiber, resin production, wastewater treatment, semiconductors, textiles, and other industries. As an important chemical raw material, it is flammable and toxic. Improper handling or transport can lead to significant safety risks. This article outlines the main uses and hazards of AN (acrylonitrile), along with proper storage practices to help you manage this substance safely.
1. What is Acrylonitrile? Hazard Classification and Main Applications
Acrylonitrile (AN) is an organic nitrile derived from propylene.
- HS Code: 2926 1000 005
- Chemical formula : C₃H₃N, Molecular structure : CH₂=CH-C≡N
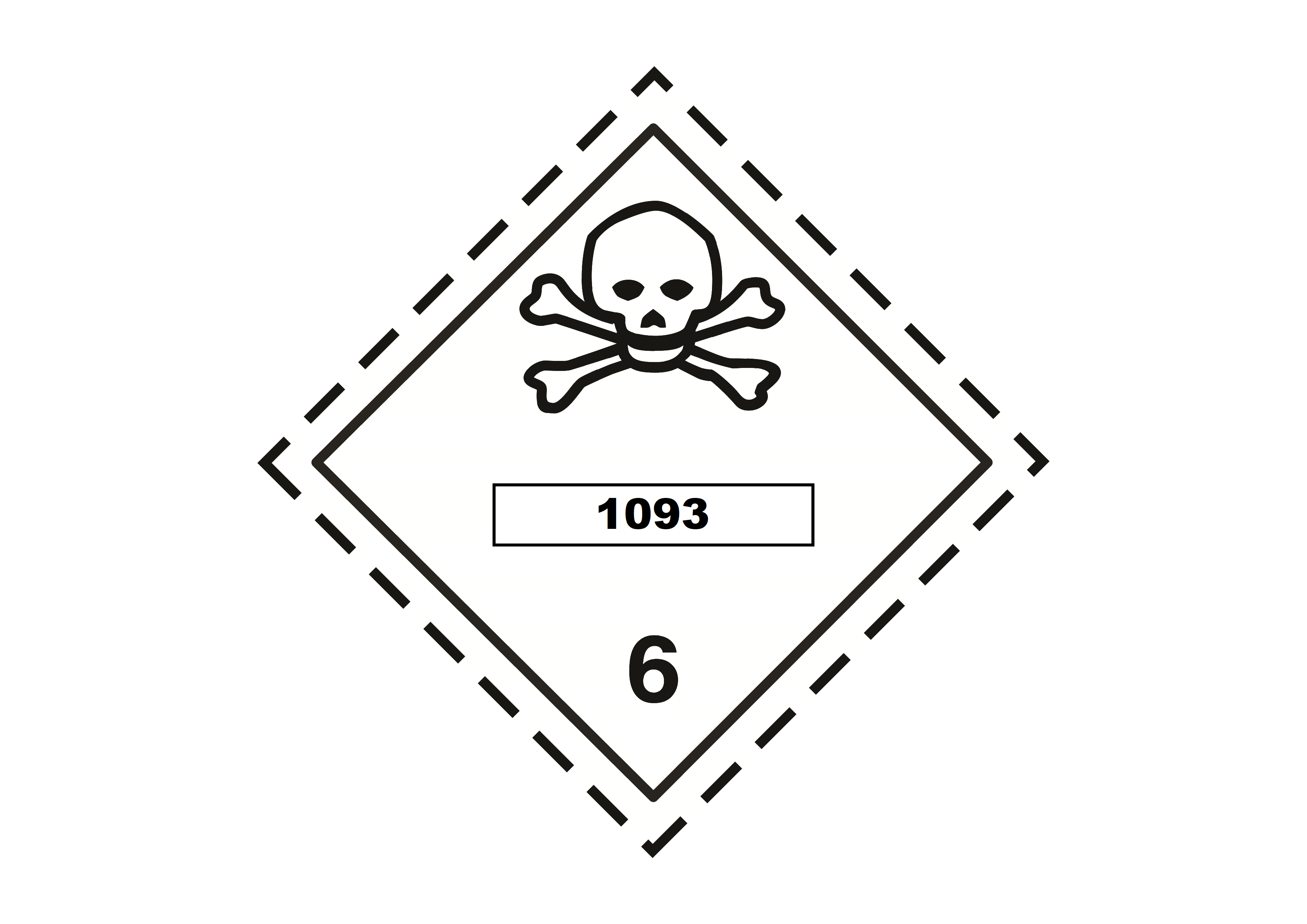
- UN Number: UN 1093
- Hazard Classes (UN): Class 3 (Flammable Liquid) & Class 6.1 (Toxic Substances)

Physical Properties :
- Boiling Point : Approx. 77°C
- Density : Approx. 0.806 g/cm³ at 20°C
- Solubility : Easily soluble in ethanol and ether; slightly soluble in water
Chemical Properties :
- Highly reactive due to a carbon-carbon double bond and a nitrile group.
- Can undergo polymerization (e.g., radical polymerization) to form polyacrylonitrile (PAN).
- Can react with water, alcohols, and other compounds.
The double and triple bonds between carbon and nitrogen make AN highly reactive, suitable for forming polymers or acting as a chemical intermediate. Here are its main uses :
(1) Monomer for Polymers
Thanks to its unsaturated chemical bonds, AN can polymerize to form polyacrylonitrile (PAN)—a precursor to carbon fiber. PAN is corrosion-resistant, heat-resistant, radiation-proof, lightweight, and strong. It’s used in:
- Vehicle chassis
- Aircraft composite materials
- Wind turbine blades
AN can also copolymerize with compounds like butadiene, styrene, vinyl acetate, and methyl acrylate to produce:
- ABS resin
- SAN resin
- Epoxy resins
- Acrylic adhesives
- Nitrile rubber
- Acrylic fibers
- Polyether polyols
Applications span plastics, textiles, chemicals, semiconductors, and polymer materials.
(2) Intermediate for Propylene Derivatives
With its highly active double bonds and nitrile group, AN reacts easily with other chemicals to form derivatives like:
- Acrylamide
- Acrylic acid
- Ammonium acrylate
- Methyl acrylate
- Adiponitrile
- Propionitrile
- Propylamine
These derivatives are important in material production, catalysis, and organic synthesis.
(3) Polymer Flocculants
Acrylamide (AM) is a derivative of acrylonitrile (AN) that can undergo polymerization to produce polyacrylamide (PAM). PAM is widely used for its ability to:
- Adsorb suspended particles in water, making it an effective agent for wastewater treatment.
- Induce secondary aggregation of small molecules, forming heavier macromolecules that settle impurities and clarify water.
- Interact with ions or other compounds, thanks to its long molecular chains and abundant amide groups that exhibit high reactivity and charge density.
These properties allow PAM to be used not only in wastewater treatment, but also as:
- A soil conditioner to improve soil structure and water retention.
- A gel-forming agent in various applications.
- A crosslinking agent and intermediate in the synthesis of other polymers.
PAM plays a vital role in environmental engineering, agriculture, and chemical manufacturing, making it an indispensable material in modern industrial processes.
2. Understanding the Hazards of AN
AN is flammable and reactive. When it reacts with incompatible substances, it can ignite, release heat, or splash. The reaction may emit toxic gases such as hydrogen cyanide (HCN), carbon monoxide (CO), and carbon dioxide (CO₂). AN is toxic to humans, with possible effects including :
- Skin contact : Blisters, allergic dermatitis.
- Ingestion : Sore throat, breathing difficulty, vomiting, abdominal pain; can be fatal.
- Eye contact : Severe irritation, burns, corneal damage, potential blindness.
- Inhalation : Cyanide disrupts oxygen utilization in the body, causing hypoxia, headache, blurred vision, vomiting, tremors, diarrhea, lack of coordination, and liver dysfunction.
3. Proper Storage Guidelines for Acrylonitrile
(1) Store in Cool, Well-Ventilated Areas
- AN should be stored away from direct sunlight, as it is photosensitive.
- Use inhibitors to prevent spontaneous polymerization.
- Keep away from oxidizers, peroxides, acids (sulfuric, hydrochloric, nitric), alkalis (sodium, potassium hydroxide), amines, nitriles, copper/aluminum metals and alloys, halogens (chlorine, bromine, iodine), and their compounds, as these can trigger explosive or exothermic reactions.
(2) Use Airtight Packaging
- Without inhibitors, AN can polymerize upon exposure to air, increasing internal pressure and possibly causing the container to rupture.
- Since it is toxic, sealed containers made of opaque material are recommended to prevent vapor leakage and light exposure.
(3) Label Containers with Hazard Signs
- Containers must be labeled clearly to inform handlers of chemical characteristics and precautions.
- Labels help first responders take appropriate action in case of emergency during transport.
Further Reading : What Are Dangerous Goods? A Quick Guide to Hazard Classification and Transport Regulations
(4) Provide Personal Protective Equipment (PPE)
- Staff handling AN must wear gloves, protective clothing, and gas masks.
- Operators should receive professional training.
(5) Avoid Spark-Generating Tools
- AN can ignite or explode upon contact with open flame, high temperature, or static electricity.
- Avoid using metal tools; opt for plastic tools in spark-free environments.
(6) Install Emergency Response Equipment
- Emergency response facilities should be available in storage areas.
- MSDS (Material Safety Data Sheets) must be easily accessible.
Further Reading : How to Understand the MSDS (Material Safety Data Sheet) and Key Details to Watch For
Published Date : January 6, 2025
