What is Epichlorohydrin ( ECH )? Hazard Classification, Uses, Risks, and Storage Guidelines
Epichlorohydrin (ECH) is widely used in the production of resins, glycerin, organic synthesis materials, and solvents. It’s an essential industrial chemical but is also highly flammable. Improper storage or transport may lead to serious harm or financial loss. This article will walk you through ECH’s key applications, hazards, and safe storage practices.
1. What Is Epichlorohydrin? Hazard Classification & Key Applications
Epichlorohydrin (ECH)
- Chemical formula : CH₂CHOCH₂Cl
- HS Code : 2910 3000 009
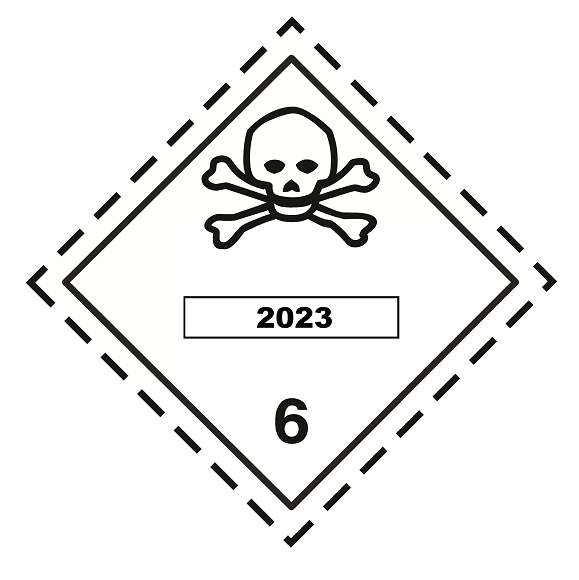
- UN Number : 2023
- UN Class : 3 (Flammable liquids) & 6.1 (Toxic substances)

Physical Properties :
- Boiling point : 115°C
- Melting point : -48°C
- Density : 1.18 g/cm³ (at 20°C)
- Solubility : Reacts exothermically with water
- Volatility : Low vapor pressure, not easily volatile
Chemical Properties :
ECH can undergo exothermic reactions when heated or when in contact with oxidizing agents, peroxides, or water—posing a risk of explosion.
It reacts with strong acids, strong bases, metals like zinc, aluminum, and iron, chlorinated compounds, amines, anilines, alkoxides, and halogens.
Explosive reactions may occur when exposed to isopropylamine, trichloroethylene, or potassium methallylate.
Categories :
ECH belongs to epoxides, organochlorines, and cyclic ethers.
Synthesis Routes :
- Allyl chloride + Hypochlorous acid → Dichloropropanol isomers + NaOH → ECH + NaCl + H₂O
- Propylene + Chlorine → ECH
Main Applications :
(1) Polymer Monomer
ECH reacts with bisphenol A to produce Bisphenol A diglycidyl ether, a precursor to epoxy resins. These resins are used in coatings, adhesives, composite materials, industrial molds, crosslinking agents, pigments, sponges, and ion-exchange resins.
(2) Organic Solvent
ECH is miscible with many polar organic solvents and can dissolve fats and some fibers, making it useful as a solvent, adhesive, textile softener, or surfactant.
(3) Derivatives
ECH decomposes in water with an exothermic reaction to produce glycerol—a key intermediate in industrial synthesis. Glycerol can be used to make explosives like nitroglycerin or react with fatty acids to form triglycerides, which are then saponified into soap using bases like sodium hydroxide.
2. Hazards of ECH
ECH is highly reactive and may catch fire or explode when exposed to incompatible substances, releasing toxic fumes and gases. It is harmful to human health, causing irritation to eyes, skin, and respiratory tract. Seek medical attention immediately upon exposure.
Health Hazards :
- Inhalation: Headache, difficulty breathing, CNS depression, reproductive harm, possibly fatal.
- Eyes: Severe irritation
- Skin: Discoloration, blistering, ulceration
- Ingestion: Nausea, vomiting, coughing
3. How to Store ECH Safely: Best Practices
(1) Store in Cool, Well-Ventilated Areas
Epichlorohydrin (ECH) should be stored in a cool, well-ventilated area. Containers must be tightly sealed and made of opaque materials to prevent exposure to sunlight. Direct sunlight or contact with air may lead to the formation of explosive chlorinated organic compounds.
ECH is highly reactive and must be kept away from the following materials to prevent combustion, heat-releasing reactions, or explosions:
- Strong acids : e.g., sulfuric acid, hydrochloric acid, nitric acid
- Strong bases : e.g., sodium hydroxide, potassium hydroxide, alcoholates
- Oxidizing agents and peroxides
- Metals : e.g., zinc, aluminum, iron and their respective chlorides
- Halogens : e.g., chlorine, bromine, iodine
- Water
- Amines and aniline compounds : e.g., isopropylamine
- Halogenated olefins : e.g., trichloroethylene
- Alkoxides and alcoholates : such as potassium methallylate, potassium methoxide, sodium methoxide, lithium methoxide, titanium butoxide, sodium butoxide, titanium isopropoxide, aluminum isopropoxide
These materials may trigger dangerous reactions when in contact with ECH. For example:
- ECH reacts violently with water and isopropylamine, releasing intense heat and splashing liquids.
- ECH and trichloroethylene can react to produce explosive dichloroacetylene.
- ECH and potassium methallylate may ignite spontaneously.
To prevent accidents, ECH should be stored separately from all the substances listed above. Proper isolation is critical to minimizing safety risks.
(2) Ensure Airtight Packaging
Epichlorohydrin (ECH) must be stored in tightly sealed containers. In the absence of stabilizers or inhibitors, ECH is prone to polymerization when exposed to air or light, which can lead to a rapid increase in internal pressure and may even cause the container to rupture or explode.
In addition to its high flammability, ECH is classified as a toxic and carcinogenic chemical. Any leakage can pose serious health risks to workers and the surrounding environment. Therefore, it’s essential that ECH is stored in a sealed container.
(3) Use Proper Hazard Labeling
Containers must carry appropriate hazard labels to inform handlers of potential dangers and emergency procedures. Labels help responders act quickly in the event of an incident during transport.
Further Reading : What Are Dangerous Goods? A Quick Guide to Hazard Classification and Transport Regulations
(4) Personnel Must Use Protective Gear
Due to its carcinogenicity and toxicity, handlers should wear gloves, protective clothing, goggles, gas masks, and SCBA (self-contained breathing apparatus), and receive professional training.
(5) Avoid Spark-Generating Tools
ECH is highly flammable and can release chlorine gas when reacting with fire, heat, static electricity, light, or steam. Use non-metal, spark-proof tools (e.g., plastic tools) in storage, handling, and transport.
(6) Equip Storage Areas with Emergency Equipment
Install emergency response equipment in areas where ECH is stored. Ensure MSDS and emergency tools are clearly marked and easily accessible.
Further Reading : How to Understand the MSDS (Material Safety Data Sheet) and Key Details to Watch For
Published Date : January 10, 2025
